|
- Samsung Biologics showcases new innovative development platforms – S-AfuCHOTM and S-OptiChargeTM – at BioProcess International 2024
- New technology platforms to proactively address evolving industry trends and enable high-quality development
INCHEON, South Korea, Sept. 25, 2024 /PRNewswire/ — Samsung Biologics (KRX: 207940.KS), a global contract development and manufacturing organization (CDMO), launched today a series of proprietary development platforms as part of continued efforts to ensure high-quality development and provide client-tailored services.
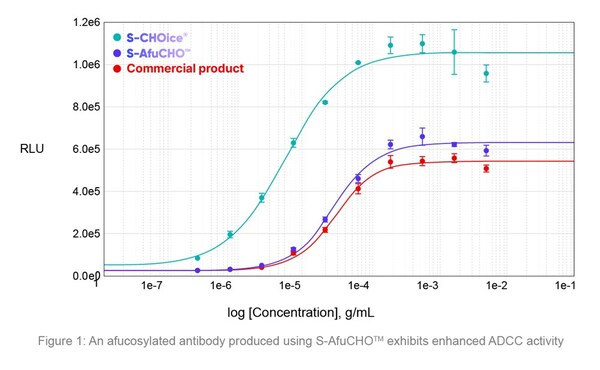
S-AfuCHOTM, introduced at BioProcess International 2024, is a cell line platform that can generate afucosylated antibodies that exhibit enhanced antibody-dependent cellular cytotoxity (ADCC) activity for increased therapeutic efficacy. The platform removes the core fucose from Fc N-glycans through knockout of the host (FUT8) gene to achieve higher ADCC activity while retaining desired titers, stability, and product quality (Figure 1).
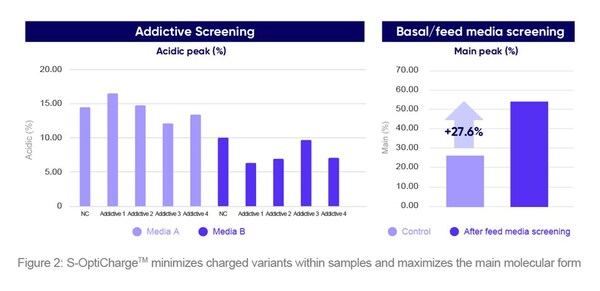
S-OptiChargeTM is an upstream process platform that can modulate a molecule’s charge variant distribution. Modifications can occur at any step of the manufacturing process, impacting product safety and efficacy. The platform minimizes charged variants within samples while maximizing the main molecular form to ensure safe therapeutic effects. By optimizing media, additives, and process parameters and using a thorough screening procedure, S-OptiChargeTM ensures high product quality through tight control over critical quality attributes to achieve optimal charge variant distribution (Figure 2).
“Our new platforms are expected to provide clients with high-performing solutions for their molecules to reduce risks and support successful development,” said Jahoon Kang, Vice President of CDO at Samsung Biologics. “We will continue to develop innovative solutions to proactively address the evolving needs of our clients and meet increased demand for complex and high-concentration biopharmaceuticals.”
Samsung Biologics is currently showcasing its latest CDO capabilities and expansion plans for Bio Campus II at BPI Boston that runs through September 26. For further information, please get in touch with a representative at booth #1025 or visit https://samsungbiologics.com/services/cdo/process-development.
About Samsung Biologics Co., Ltd.
Samsung Biologics (KRX: 207940.KS) is a fully integrated, end-to-end CDMO service provider, offering seamless development and manufacturing solutions from cell line development to final aseptic fill/finish as well as laboratory testing support for the biopharmaceutical products we manufacture. Our state-of-the-art facilities are CGMP compliant with bioreactors ranging from small to large scales to serve varying client needs. To maximize our operational efficiency and expand our capabilities in response to growing biomanufacturing demand, Samsung Biologics completed Bio Campus I with Plant 4, offering a combined 604 kL total capacity, and launched Bio Campus II with the construction of Plant 5, which will be operational in April 2025, adding 180 kL biomanufacturing capacity. Additionally, Samsung Biologics America enables the company to work in closer proximity to clients based in the U.S. and Europe. We continue to upgrade our capabilities to accommodate our clients by investing in technologies such as an antibody-drug conjugate (ADC) facility, a dedicated mRNA manufacturing facility, and additional aseptic filling capacity. As a sustainable CDMO partner of choice, we are committed to on-time, in-full delivery of the products we manufacture with our flexible manufacturing solutions, operational excellence, and proven expertise.
Samsung Biologics Media Contact
Claire Kim, Head of Global Marketing & Communications
cair.kim@samsung.com